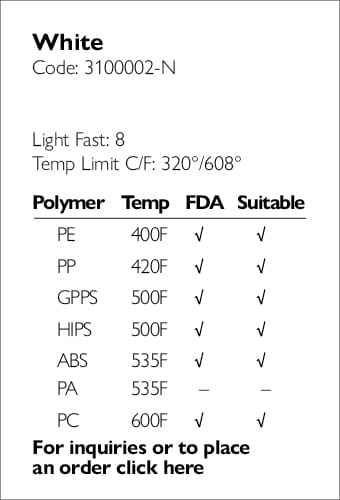
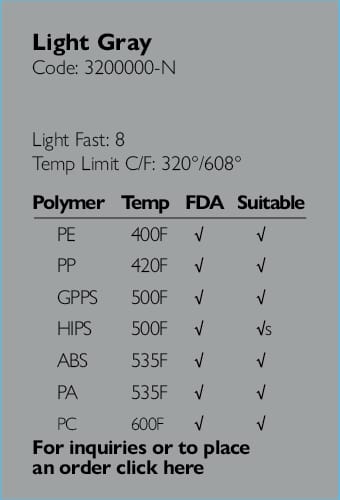
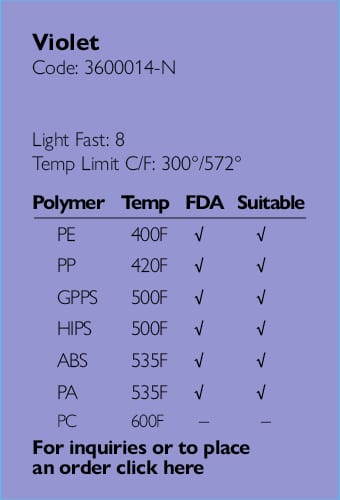
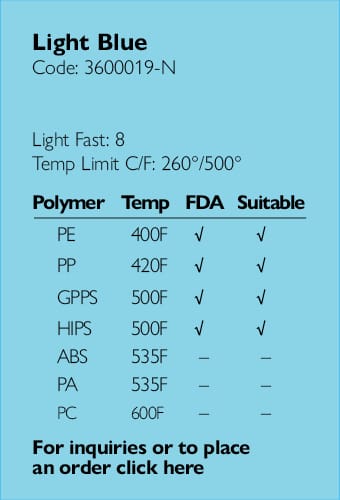
Providing full regulatory and compliance consultation services.
Regulatory Status Documents 1-3 Days
Safety Data Sheets 1-3 Days
Food Contact Compliance 1-3 Days
Heavy Metals Declarations (CONEG / RoHS etc.) 1-3 Days
Agency Submissions
NSF: Direct Response to Customer
DMF: FDA registered product, 2 days
Health Canada: Minimum 6 months
Absence/Presence Statements 1-3 Days
National Inventories 1-3 Days
Customer Questionnaires 1-3 Days
SVHC – REACH 1-3 Days
Allergens 1-3 Days
Compliance with Specific Segments Legislations 1-3 Days
North America
Regulatory Affairs
Terre Haute, IN USA
+1 812 466 9828
RegulatoryNorthAmerica@ampacet.com
South America
Regulatory Affairs
Buenos Aires, Argentina
Tel: +54 11 4110 4200
RegulatorySouthAmerica@ampacet.com
Asia-Pacific
Regulatory Affairs
Rayong, Thailand
Tel: +66 38 927 999
RegulatoryAsia@ampacet.com
Click here for more detail on Ampacet’s regulatory and compliance services.