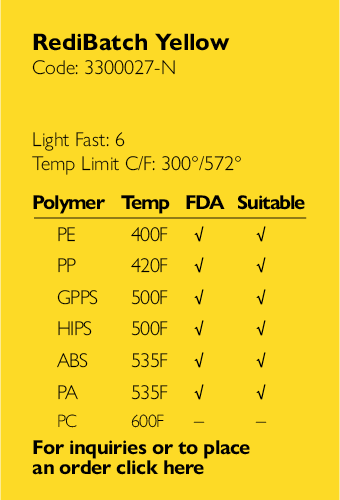
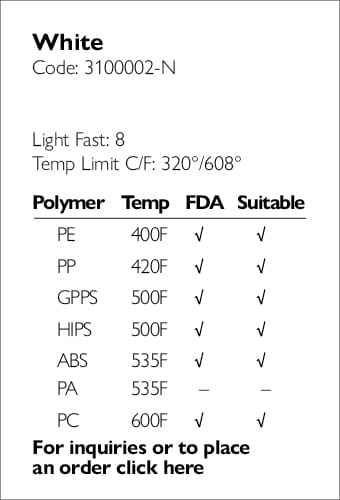
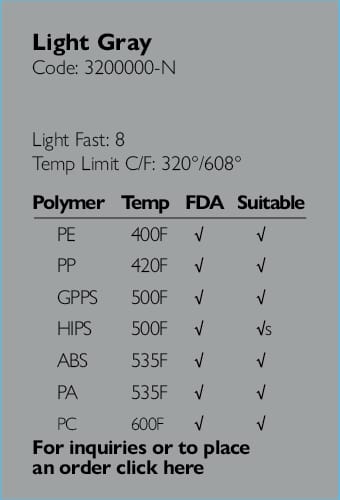
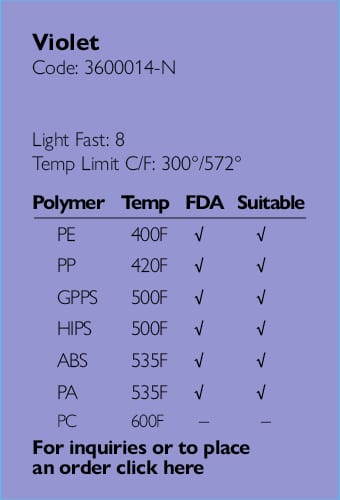
Ampacet, a global masterbatch leader, has introduced ProVital™+ Gamma-Protect, a medical-grade additive available for the European market that has been designed to preserve mechanical and optical properties of polypropylene-based medical and pharmaceutical articles during gamma and e-beam sterilization processes.

Ampacet ProVital™+ Gamma-Protect is a medical-grade additive for the European market that has been designed to preserve mechanical and optical properties of polypropylene-based medical and pharmaceutical articles during gamma and e-beam sterilization processes.
Gamma and e-beam sterilization techniques are commonly used by the medical industry to eliminate microorganisms such as bacteria, viruses and spores from medical devices, in-vitro diagnostic equipment and packaging systems. However, gamma and e-beam sterilization methods are known to alter the optical and mechanical properties of the polymers, including elongation, tensile and impact strength.
Ampacet ProVital™+ Gamma-Protect is an efficient medical grade additive solution that stabilizes polypropylene during gamma or e-beam sterilization processes and helps to preserve mechanical and optical properties and prevent degradation of the polymer. Applications include medical devices, in-vitro diagnostic equipment and pharmaceutical packaging systems.
Ampacet ProVital™+ Gamma-Protect has also been pre-evaluated for biocompatibility according to ISO 10993 part 5, 10 and 23 (cytotoxicity, sensitization and irritation).
Ampacet ProVital™+ Gamma-Protect provides full consistency of formulation with a no-change policy with manufacturing under consistent process parameters.
The ProVital+ product range is available for the European market. For more information on the Ampacet ProVital+ range, email marketing.europe@ampacet.com